- ⌂
-
Le DPM
Les Equipements
L'Environnement
Zoom sur... Le Bâtiment André Rassat
Nommé d'après une figure tutélaire de la chimie grenobloise, ce bâtiment est recouvert d’une double peau en feuille métallique qui apporte une protection thermique sur 3 côtés et crée une unité architecturale favorisant l'intégration parmi les arbres du site.
-
Thématiques
Le Thème
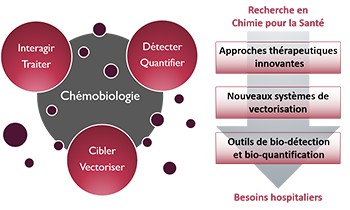
« Approches thérapeutiques innovantes »De nouvelles solutions thérapeutiques, de la cible biomacromolécu-laire émergente aux approches originales pour traiter les maladies
Le Thème
« Nouveaux systèmes de vectorisation »Combiner les propriétés d’inclusion de principes actifs, de franchisse-ment de barrières, d’adressage et de relar-gage en milieu vivant
Le Thème
« Outils de bio-détection et bio-quantification »Des dispositifs analytiques originaux pour la détection de cibles, de l’ion au micro-organisme en milieu complexe
Zoom sur... La Chémobiologie
-
Équipes
L'Équipe « COMET »
« COMET » développe la conception rationnelle, la synthèse et/ou l'extraction de composés à forte diversité/complexité comme nouveaux agents thérapeutiques et outils moléculaires pour la pénétration cellulaire ou la détection de biomolécules, actifs in vivo.L'Équipe « NOVA »
« NOVA » utilise des acides nucléiques fonctionnels comme éléments de reconnaissance pour des applications thérapeutiques ou diagnostiques, comme la sélection d'oligonucléotides, ou le développement de dispositifs d'analyses et de nanovecteurs.Les Services
-
Productions
Les Publications
La Vulgarisation
Les JSM
Zoom sur... La 12ème JSM (15 juin 2023)
Le DPM organise des journées scientifiques consacrées au médicament. L'objectif est de rassembler les spécialistes académiques et industriels autour d'une thématique. 2023 : Apports de la Chimie Click et de la Lumière en Chemobiologie
-
Partenariats
Les Formations
Les Consortiums
Les Financements
Zoom sur... L'environnement Grenoblois
Le DPM est un acteur central sur le bassin grenoblois en chimie, biologie et santé, lié au CHU Grenoble Alpes et à de nombreuses autres organisations : Pole de Recherche CBS, ICMG, Labex ARCANE, EUR CBH, Institut Carnot Polynat, Réseau GREEN.
Article
- Projet
- Isabelle BAUSSANNE, Jean-Luc DECOUT,
- Titre
- Tuning the antibacterial activity of amphiphilic neamine derivatives and comparison to paromamine homologues.
-
[Full paper
 ]
] - Auteurs
- L. Zimmermann, A. Bussiere, M. Ouberai, I. Baussanne, C. Jolivalt, M.-P. Mingeot-Leclercq, J.-L. Decout.
- Edition
- J. Med. Chem. 2013, 56, 7691-7705.
- Année
- 2013
- Résumé
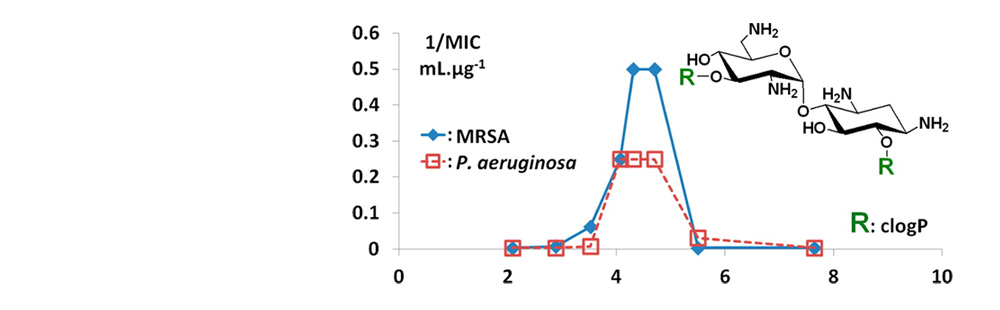
- Aminoglycosides are antibiotic drugs that act through binding to rRNA. In the search for antimicrobial amphiphilic aminoglycosides targeting bacterial membranes, we report here on the discovery of three dialkyl derivatives of the small aminoglycoside neamine active against susceptible and resistant Gram-positive and Gram-negative bacteria. One of these derivatives (R = 2-naphthylpropyl), which has good activity against MRSA and VRSA, showed a low toxicity in eukaryotic cells at 10 μM. The synthesis of amphiphilic paromamine and neamine homologous derivatives pointed out the role of the 6′-amine function of the neamine core in the antibacterial effects. The optimal number of lipophilic substituents to be attached to the neamine core and the corresponding required lipophilicity determined here should permit a more selective targeting of bacterial membranes relative to eukaryotic membranes. This work revealed the existence of windows of lipophilicity necessary for obtaining strong antibacterial effects that should be of interest in the field of antibacterial amphiphilic aminoglycosides.




 Annuaire
Annuaire Contact
Contact Plan d'accès
Plan d'accès ENG
ENG Login
Login


