- ⌂
-
Le DPM
Les Equipements
L'Environnement
Zoom sur... Le Bâtiment André Rassat
Nommé d'après une figure tutélaire de la chimie grenobloise, ce bâtiment est recouvert d’une double peau en feuille métallique qui apporte une protection thermique sur 3 côtés et crée une unité architecturale favorisant l'intégration parmi les arbres du site.
-
Thématiques
Le Thème
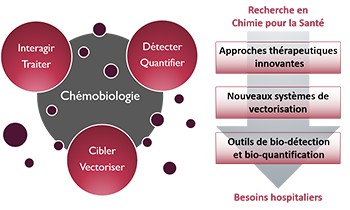
« Approches thérapeutiques innovantes »De nouvelles solutions thérapeutiques, de la cible biomacromolécu-laire émergente aux approches originales pour traiter les maladies
Le Thème
« Nouveaux systèmes de vectorisation »Combiner les propriétés d’inclusion de principes actifs, de franchisse-ment de barrières, d’adressage et de relar-gage en milieu vivant
Le Thème
« Outils de bio-détection et bio-quantification »Des dispositifs analytiques originaux pour la détection de cibles, de l’ion au micro-organisme en milieu complexe
Zoom sur... La Chémobiologie
-
Équipes
L'Équipe « COMET »
« COMET » développe la conception rationnelle, la synthèse et/ou l'extraction de composés à forte diversité/complexité comme nouveaux agents thérapeutiques et outils moléculaires pour la pénétration cellulaire ou la détection de biomolécules, actifs in vivo.L'Équipe « NOVA »
« NOVA » utilise des acides nucléiques fonctionnels comme éléments de reconnaissance pour des applications thérapeutiques ou diagnostiques, comme la sélection d'oligonucléotides, ou le développement de dispositifs d'analyses et de nanovecteurs.Les Services
-
Productions
Les Publications
La Vulgarisation
Les JSM
Zoom sur... La 12ème JSM (15 juin 2023)
Le DPM organise des journées scientifiques consacrées au médicament. L'objectif est de rassembler les spécialistes académiques et industriels autour d'une thématique. 2023 : Apports de la Chimie Click et de la Lumière en Chemobiologie
-
Partenariats
Les Formations
Les Consortiums
Les Financements
Zoom sur... L'environnement Grenoblois
Le DPM est un acteur central sur le bassin grenoblois en chimie, biologie et santé, lié au CHU Grenoble Alpes et à de nombreuses autres organisations : Pole de Recherche CBS, ICMG, Labex ARCANE, EUR CBH, Institut Carnot Polynat, Réseau GREEN.
Article
- Projet
- Benjamin BOUCHERLE,
- Titre
- Structure-Based Design of PDZ Ligands as Inhibitors of 5-HT2A Receptor/PSD-95 PDZ1 Domain Interaction Possessing Anti-hyperalgesic Activity
-
[Full paper
 ]
] - Auteurs
- Alexandre Vogrig, Liam Dorr, Naoual Bouzidi, Benjamin Boucherle, Anne-Sophie Wattiez, Elisabeth Cassier, Gary Vallon, Isabelle Ripoche, Isabelle Abrunhosa-Thomas, Philippe Marin, Lionel Nauton, Vincent Thery, Christine Courteix, Lu-Yun Lian, Sylvie Ducki
- Edition
- ACS Chemical Biology 07/2013; 8(10), 2209 − 2216
- Année
- 2013
- Résumé
- Disrupting the interaction between the PDZ protein PSD-95 and the C-terminal domain of the 5-HT2A serotonin receptor has been shown to reduce hyperalgesia in a rodent model of neuropathic pain. Here, we designed, and synthesized PDZ ligands capable of binding to the first PDZ domain (PDZ1) of PSD-95 protein and evaluated their biological activity in vitro and in vivo. A series of substituted indoles were identified by docking simulations, and six novel analogues were synthesized. Three analogues displayed strong interactions with the first PDZ domain (PDZ1) of PDZ-95 in 1H-15N NMR HSQC experiments and two of them were actually able to inhibit the interaction between PSD-95 and the 5-HT2A receptor in vitro. We identified compound 8b as the analogue able to significantly suppress mechanical hyperalgesia in an experimental model of traumatic neuropathic pain in the rat. This effect was suppressed by the co-administration of the 5-HT2A receptor antagonist M100907, consistent with an inhibitory effect upon 5-HT2A receptor/PSD-95 interaction. Finally, we determined an NMR-restraint driven model structure for the PSD95 PDZ1/8b complex, which confirms that indole 8b binds to the putative PDZ-ligand binding site.




 Annuaire
Annuaire Contact
Contact Plan d'accès
Plan d'accès ENG
ENG Login
Login


