- ⌂
-
Le DPM
Les Equipements
L'Environnement
Zoom sur... Le Bâtiment André Rassat
Nommé d'après une figure tutélaire de la chimie grenobloise, ce bâtiment est recouvert d’une double peau en feuille métallique qui apporte une protection thermique sur 3 côtés et crée une unité architecturale favorisant l'intégration parmi les arbres du site.
-
Thématiques
Le Thème
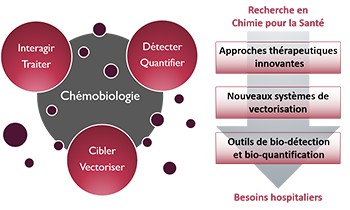
« Approches thérapeutiques innovantes »De nouvelles solutions thérapeutiques, de la cible biomacromolécu-laire émergente aux approches originales pour traiter les maladies
Le Thème
« Nouveaux systèmes de vectorisation »Combiner les propriétés d’inclusion de principes actifs, de franchisse-ment de barrières, d’adressage et de relar-gage en milieu vivant
Le Thème
« Outils de bio-détection et bio-quantification »Des dispositifs analytiques originaux pour la détection de cibles, de l’ion au micro-organisme en milieu complexe
Zoom sur... La Chémobiologie
-
Équipes
L'Équipe « COMET »
« COMET » développe la conception rationnelle, la synthèse et/ou l'extraction de composés à forte diversité/complexité comme nouveaux agents thérapeutiques et outils moléculaires pour la pénétration cellulaire ou la détection de biomolécules, actifs in vivo.L'Équipe « NOVA »
« NOVA » utilise des acides nucléiques fonctionnels comme éléments de reconnaissance pour des applications thérapeutiques ou diagnostiques, comme la sélection d'oligonucléotides, ou le développement de dispositifs d'analyses et de nanovecteurs.Les Services
-
Productions
Les Publications
La Vulgarisation
Les JSM
Zoom sur... La 12ème JSM (15 juin 2023)
Le DPM organise des journées scientifiques consacrées au médicament. L'objectif est de rassembler les spécialistes académiques et industriels autour d'une thématique. 2023 : Apports de la Chimie Click et de la Lumière en Chemobiologie
-
Partenariats
Les Formations
Les Consortiums
Les Financements
Zoom sur... L'environnement Grenoblois
Le DPM est un acteur central sur le bassin grenoblois en chimie, biologie et santé, lié au CHU Grenoble Alpes et à de nombreuses autres organisations : Pole de Recherche CBS, ICMG, Labex ARCANE, EUR CBH, Institut Carnot Polynat, Réseau GREEN.
Article
- Projet
- Farid OUKACINE,
- Titre
- An improved capillary electrophoresis method for in vitro monitoring of the challenging early steps of A beta(1-42) peptide oligomerization: Application to anti-Alzheimer's drug discovery
-
[Full paper
 ]
] - Auteurs
- D. Brinet, J. Kaffy, F. Oukacine, S. Glumm, S. Ongeri, M. Taverna
- Edition
- Electrophoresis 2014, 35, 3302-3309.
- Année
- 2014
- Résumé
- We report an improved CE method to monitor in vitro the self-assembly of monomeric amyloid beta-peptide (42 amino acids amyloid beta-peptide, A beta(1-42)) and in particular the crucial early steps involved in the formation of the neurotoxic oligomers. In order to start the kinetics from the beginning, sample preparation was optimized to provide samples containing exclusively the monomeric form. The CE method was also improved using a dynamic coating and by reducing the separation distance. Using this method, the disappearance of the monomer as well as the progressive formation of four species during the self-assembly process can now be monitored and quantified over time. The hydrodynamic radius of the species present at the initial kinetics step was estimated around 1.8 nm by Taylor dispersion analysis while SDS-PAGE analyses showed the predominance of the monomer. These results confirmed that the A beta(1-42) species present at this initial time was the monomer. Methylene blue, an anti-Alzheimer disease candidate, was then evaluated. In spite of an oligomerization inhibition, the enhanced disappearance of the A beta(1-42) monomer provoked by methylene blue was demonstrated for the first time. This method, allowing the monomeric and smallest oligomeric species to be monitored, represents a new accurate and precise way to evaluate compounds for drug discovery.




 Annuaire
Annuaire Contact
Contact Plan d'accès
Plan d'accès ENG
ENG Login
Login


