- ⌂
-
Le DPM
Les Equipements
L'Environnement
Zoom sur... Le Bâtiment André Rassat
Nommé d'après une figure tutélaire de la chimie grenobloise, ce bâtiment est recouvert d’une double peau en feuille métallique qui apporte une protection thermique sur 3 côtés et crée une unité architecturale favorisant l'intégration parmi les arbres du site.
-
Thématiques
Le Thème
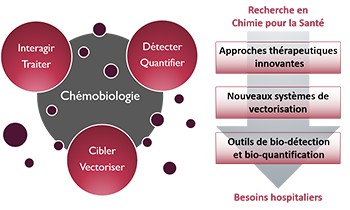
« Approches thérapeutiques innovantes »De nouvelles solutions thérapeutiques, de la cible biomacromolécu-laire émergente aux approches originales pour traiter les maladies
Le Thème
« Nouveaux systèmes de vectorisation »Combiner les propriétés d’inclusion de principes actifs, de franchisse-ment de barrières, d’adressage et de relar-gage en milieu vivant
Le Thème
« Outils de bio-détection et bio-quantification »Des dispositifs analytiques originaux pour la détection de cibles, de l’ion au micro-organisme en milieu complexe
Zoom sur... La Chémobiologie
-
Équipes
L'Équipe « COMET »
« COMET » développe la conception rationnelle, la synthèse et/ou l'extraction de composés à forte diversité/complexité comme nouveaux agents thérapeutiques et outils moléculaires pour la pénétration cellulaire ou la détection de biomolécules, actifs in vivo.L'Équipe « NOVA »
« NOVA » utilise des acides nucléiques fonctionnels comme éléments de reconnaissance pour des applications thérapeutiques ou diagnostiques, comme la sélection d'oligonucléotides, ou le développement de dispositifs d'analyses et de nanovecteurs.Les Services
-
Productions
Les Publications
La Vulgarisation
Les JSM
Zoom sur... La 12ème JSM (15 juin 2023)
Le DPM organise des journées scientifiques consacrées au médicament. L'objectif est de rassembler les spécialistes académiques et industriels autour d'une thématique. 2023 : Apports de la Chimie Click et de la Lumière en Chemobiologie
-
Partenariats
Les Formations
Les Consortiums
Les Financements
Zoom sur... L'environnement Grenoblois
Le DPM est un acteur central sur le bassin grenoblois en chimie, biologie et santé, lié au CHU Grenoble Alpes et à de nombreuses autres organisations : Pole de Recherche CBS, ICMG, Labex ARCANE, EUR CBH, Institut Carnot Polynat, Réseau GREEN.
Article
- Projet
- Farid OUKACINE,
- Titre
- Study of interactions between oppositely charged dendrigraft poly-L-lysine and human serum albumin by continuous frontal analysis capillary electrophoresis and fluorescence spectroscopy
-
[Full paper
 ]
] - Auteurs
- N. Sisavath, L. Leclercq, T. Le Saux, F. Oukacine, H. Cottet
- Edition
- J. Chromatogr. A 2013, 1289, 127-132.
- Année
- 2013
- Résumé
- Dendrigraft poly-L-lysine (DGL) are biomacromolecules of great interest for many applications including antibacterial activity, drug delivery systems, gene therapy and production of antibodies. As human serum albumin (HSA) is the most abundant serum protein, the study of interactions between these two compounds is crucial for the use of DGL in drug or gene delivery systems. The present work aims at determining the number of binding sites and the corresponding successive equilibrium constants between DGL of generation 3 (G3) and HSA in physiological conditions. To meet this end, continuous frontal analysis capillary electrophoresis (FACCE) and fluorescence spectroscopic methods were implemented and compared. FACCE was performed on a polycationic modified capillary in combination with a co-pressure that allowed for selectively introducing the free G3 from the G3/HSA mixtures. FACCE studies demonstrated that HSA has 2 binding sites with DGL G3 with the following successive constants K-1 = 31.2 x 10(3) M-1 and K-2 = 30.6 x 10(3) M-1. For a 1 g/L concentration in G3 and assuming a plasmatic HSA concentration of 40 g/L, these binding constants lead to only 5% free DGL in the medium. It was also shown that the interactions between G3 and HSA corresponded to a model of cooperative sites. These results are in good agreement with the presence of two negatively charged domains in the HSA. Good fitting of the fluorescence spectroscopy data was obtained using the equilibrium constants derived from FACCE. Nevertheless, due to the high number of fitting parameters, it was difficult to fit the fluorescence spectroscopic data independently of the results obtained by FACCE




 Annuaire
Annuaire Contact
Contact Plan d'accès
Plan d'accès ENG
ENG Login
Login


