- ⌂
-
Le DPM
Les Equipements
L'Environnement
Zoom sur... Le Bâtiment André Rassat
Nommé d'après une figure tutélaire de la chimie grenobloise, ce bâtiment est recouvert d’une double peau en feuille métallique qui apporte une protection thermique sur 3 côtés et crée une unité architecturale favorisant l'intégration parmi les arbres du site.
-
Thématiques
Le Thème
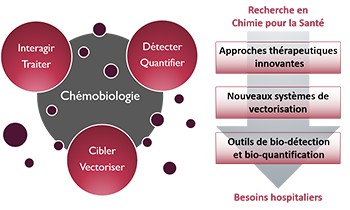
« Approches thérapeutiques innovantes »De nouvelles solutions thérapeutiques, de la cible biomacromolécu-laire émergente aux approches originales pour traiter les maladies
Le Thème
« Nouveaux systèmes de vectorisation »Combiner les propriétés d’inclusion de principes actifs, de franchisse-ment de barrières, d’adressage et de relar-gage en milieu vivant
Le Thème
« Outils de bio-détection et bio-quantification »Des dispositifs analytiques originaux pour la détection de cibles, de l’ion au micro-organisme en milieu complexe
Zoom sur... La Chémobiologie
-
Équipes
L'Équipe « COMET »
« COMET » développe la conception rationnelle, la synthèse et/ou l'extraction de composés à forte diversité/complexité comme nouveaux agents thérapeutiques et outils moléculaires pour la pénétration cellulaire ou la détection de biomolécules, actifs in vivo.L'Équipe « NOVA »
« NOVA » utilise des acides nucléiques fonctionnels comme éléments de reconnaissance pour des applications thérapeutiques ou diagnostiques, comme la sélection d'oligonucléotides, ou le développement de dispositifs d'analyses et de nanovecteurs.Les Services
-
Productions
Les Publications
La Vulgarisation
Les JSM
Zoom sur... La 12ème JSM (15 juin 2023)
Le DPM organise des journées scientifiques consacrées au médicament. L'objectif est de rassembler les spécialistes académiques et industriels autour d'une thématique. 2023 : Apports de la Chimie Click et de la Lumière en Chemobiologie
-
Partenariats
Les Formations
Les Consortiums
Les Financements
Zoom sur... L'environnement Grenoblois
Le DPM est un acteur central sur le bassin grenoblois en chimie, biologie et santé, lié au CHU Grenoble Alpes et à de nombreuses autres organisations : Pole de Recherche CBS, ICMG, Labex ARCANE, EUR CBH, Institut Carnot Polynat, Réseau GREEN.
Couvertures de journaux
-
 Front Cover • Phytochemistry, Octobre 2017, Volume 142
Front Cover • Phytochemistry, Octobre 2017, Volume 142 
Occurrences, biosynthesis and properties of aurones as high-end evolutionary products
Recent years have witnessed a considerable renewed interest for the uncommon flavonoid class of aurones. The characterization of two major biosynthetic machineries involved in their biosynthesis in flowers has encouraged the revival of phytochemical studies and identification of original structures, a process started almost seventy-five years ago. This review draws up an exhaustive map of natural occurrences of aurones their biosynthetic pathways and roles, with the aim to link their original structural properties among flavonoids to their place in evolution and the selective advantages they bring to some of the most advanced taxa in the plant kingdom. -
 Inside Cover • Chemistry, A European Journal, Avril 2017, Volume 23, Issue 21
Inside Cover • Chemistry, A European Journal, Avril 2017, Volume 23, Issue 21 
Selective luminescent labeling of DNA and RNA quadruplexes by π-extended ruthenium light-up probes
A series of RuII complexes exhibiting π-extended, acridine-based ancillary chelating heterocycles display high affinity and selectivity for DNA and RNA quadruplexes. The most promising candidates (3, 4) possess remarkable light-up luminophore properties (up to 330-fold luminescence enhancement upon interaction with quadruplexes), enabling them to discriminate quadruplexes from genomic DNA owing to a photochemical mechanism involving DNA protection against non-radiative decay (DAND), thus deviating from the other complexes of this series of ligands that exhibit an excited-state intramolecular proton transfer (ESIPT) that quenches their luminescence. The in vitro and preliminary in cellulo results shown here confirm the interest of this new family of fluorophores as invaluable molecular tools to detect G-quadruplexes in cells. -
 Front Cover • ACS Medicinal Chemistry Letters, Janvier 2017, Volume 8, Issue 1
Front Cover • ACS Medicinal Chemistry Letters, Janvier 2017, Volume 8, Issue 1 
2-Hydroxypyridine-N-oxide-embedded aurones as potent human tyrosinase inhibitors
With the aim to develop effective and selective human tyrosinase inhibitors, we investigated aurone derivatives whose B-ring was replaced by a non-oxidizable 2-hydroxypyridine-N-oxide (HOPNO) moiety. These aurones were synthesized and evaluated as inhibitors of purified human tyrosinase. Excellent inhibition activity was revealed and rationalized by theoretical calculations. The aurone backbone was especially found to play a crucial role, as the HOPNO moiety alone provided very modest activity on human tyrosinase. Furthermore, the in vitro activity was confirmed by measuring the melanogenesis suppression ability of the compounds in melanoma cell lysates and whole cells. Our study reveals that HOPNO-embedded 6-hydroxyaurone is to date the most effective inhibitor of isolated human tyrosinase. Owing to its low toxicity and its high inhibition activity, it could represent a milestone on the path toward new valuable agents in dermocosmetics, as well as in medical fields where it was recently suggested that tyrosinase could play key roles. -
 Front Cover • Natural Product Reports, Septembre 2016, Volume 33, Issue 9
Front Cover • Natural Product Reports, Septembre 2016, Volume 33, Issue 9 
Nauclea latifolia: biological activity and alkaloid phytochemistry of a West African tree
Nauclea latifolia (syn. Sarcocephalus latifolius, Rubiaceae), commonly called the African pincushion tree, is a plant widely used in folk medicine in different regions of Africa for treating a variety of illnesses, including malaria, epilepsy and pain. N. latifolia has not only drawn the interest of traditional healers but also of phytochemists, who have identified a range of bioactive indole alkaloids in its tissue. More recently, following up on the traditional use of extracts in pain management, a bio-guided purification from the roots of the tree led to the identification of the active ingredient as tramadol, available as a synthetic analgesic since the 1970s. The discovery of this compound as a natural phytochemical was highlighted worldwide. This review focuses on the correlation between extracted compounds and pharmacological activities, paying special attention to infectious diseases and neurologically-related disorders. A critical analysis of the data reported so far on the natural origin of tramadol and its proposed biosynthesis is also presented.




 Annuaire
Annuaire Contact
Contact Plan d'accès
Plan d'accès ENG
ENG Login
Login



