Article
- Projet
-
-
Eric PEYRIN, Corinne RAVELET, Sandrine PERRIER, Valérie GUIEU,
- Titre
-
Single-Stranded DNA Binding Protein-Assisted Fluorescence Polarization Aptamer Assay for Detection of Small Molecules
-
[Full paper
 ]
]
- Auteurs
- Z. Zhu, C. Ravelet, S. Perrier, V. Guieu, E. Fiore, E. Peyrin.
- Edition
- Anal. Chem. 2012, 84, 7203-7211.
- Année
- 2012
- Résumé
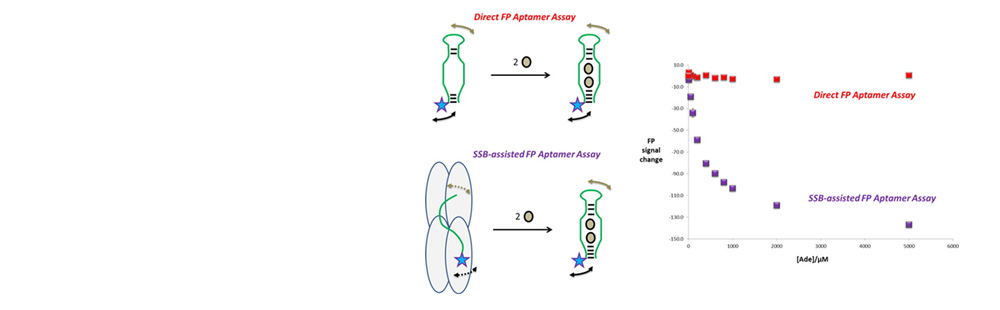
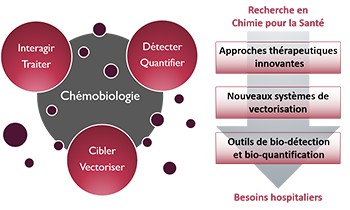
- Here, we describe a new fluorescence polarization aptamer assay (FPAA) strategy which is based on the use of the single-stranded DNA binding (SSB) protein from Escherichia coli as a strong FP signal enhancer tool. This approach relied on the unique ability of the SSB protein to bind the nucleic acid aptamer in its free state but not in its target-bound folded one. Such a feature was exploited by using the antiadenosine (Ade)-DNA aptamer (Apt-A) as a model functional nucleic acid. Two fluorophores (fluorescein and Texas Red) were introduced into different sites of Apt-A to design a dozen fluorescent tracers. In the absence of the Ade target, the binding of the labeled aptamers to SSB governed a very high fluorescence anisotropy increase (in the 0.130-0.200 range) as the consequence of (i) the large global diffusion difference between the free and SSB-bound tracers and (ii) the restricted movement of the dye in the SSB-bound state. When the analyte was introduced into the reaction system, the formation of the folded tertiary structure of the Ade-Apt-A complex triggered the release of the labeled nucleic acids from the protein, leading to a strong decrease in the fluorescence anisotropy. The key factors involved in the fluorescence anisotropy change were considered through the development of a competitive displacement model, and the optimal tracer candidate was selected for the Ade assay under buffer and realistic (dild. human serum) conditions. The SSB-assisted principle was found to operate also with another aptamer system, i.e., the antiargininamide DNA aptamer, and a different biosensing configuration, i.e., the sandwich-like design, suggesting the broad usefulness of the present approach. This sensing platform allowed generation of a fluorescence anisotropy signal for aptamer probes which did not operate under the direct format and greatly improved the assay response relative to that of the most previously reported small target FPAA.







 ]
] 



 Annuaire
Annuaire Contact
Contact Plan d'accès
Plan d'accès ENG
ENG Login
Login



