Article
- Projet
-
-
Ahcène BOUMENDJEL,
- Titre
-
A novel platinum-maurocalcine conjugate induces apoptosis of human glioblastoma cells by acting through the ROS-ERK/AKT-p53 Pathway.
-
- Auteurs
- Aroui S, Dardevet L, W. BAjmia, M. de Boisvilliers, F. Perrin, A. Laajimi, A. Boumendjel, A. Kenani, J. M. Muller, M. de Waard.
- Edition
- Mol Pharm. 2015, 12, 4336-4348.
- Année
- 2015
- Résumé
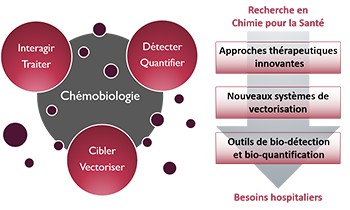
- Glioblastoma multiforme (GBM) is a highly malignant and aggressive primary brain tumor. In spite of an arsenal of therapeutic interventions, the prognosis of glioblastoma remains very poor. Cisplatin-based therapy is one of the most important chemotherapy treatments for GBM although its efficacy is limited by drug resistance and undesirable side effects. In the present study, we designed a chimera molecule containing the platinum binding moiety MBL-III-7 (1) attached N-terminal to the sequence of D-maurocalcine (D-MCa), a protease-resistant and highly efficient cell penetrating peptide derived from the Tunisian chactid scorpion toxin, L-MCa. The concept behind this design is that MCa, through its cell retention properties, should reduce cell expulsion of the platinum complex and increase its efficiency. The anticancer properties of the synthetized platinum-analogue Pt-MBL-III_7-D_MCa (Pt-1-DMCa) were assessed in human glioblastoma cells (U87) by assaying cell viability and apoptosis. The new molecule exhibited enhanced anticancer efficacy compared to cisplatin, especially at low doses. By inducing intracellular oxidative stress, Pt-1-DMCa potentiated platinum-induced DNA damage and led to enhanced p53 phosphorylation, followed by increased activation of both mitochondrial and death receptor pathways. Decreased phosphorylated AKT and ERK levels were associated with the apoptosis induced by the novel synthesized cisplatin analogue. Our results suggested that a chimera between platinum and a maurocalcine-derived cell penetrating peptide is a highly successful anticancer compound that works by targeting the intracellular redox system. Pt-1-DMCa is an interesting candidate for a preclinical assessment of platinum-based therapy in GBM treatments and possibly other cancer types. Glioblastoma multiforme (GBM) is a highly malignant and aggressive primary brain tumor. In spite of an arsenal of therapeutic interventions, the prognosis of glioblastoma remains very poor. Cisplatin-based therapy is one of the most important chemotherapy treatments for GBM although its efficacy is limited by drug resistance and undesirable side effects. Therefore, finding platinum-based alternatives to the anticancer drug cisplatin may yields drugs with improved reactivity and selectivity. In the present study, we designed an hybrid molecule containing the platinum binding moiety MBL-III-7 (1) attached N-terminal to the sequence of D-maurocalcine (D-MCa), a protease-resistant and highly efficient cell penetrating peptide derived from our knowledge of the Tunisian chactid scorpion toxin, L-MCa. The concept behind this design is that MCa has been shown to have cell retention properties which should avoid cell expulsion of the platinum complex and increase its efficiency. The anticancer properties of the synthetized platinum-analogue Pt-MBL-III_7-D_MCa (Pt-1-DMCa) were assessed in human glioblastoma cells (U87) by assaying cell viability and apoptosis. The new molecule exhibited enhanced anticancer efficacy compared to cisplatin especially at low doses. By inducing intracellular oxidative stress, Pt-1-DMCa potentiated platinum-induced DNA damage and led to enhanced p53 phosphorylation, followed by increased activation of both mitochondrial and death receptor pathways. Decreased phosphorylated AKT and ERK was associated with the apoptosis induced by the novel synthesized cisplatin analogue. Our results suggested that a chimera of platinum and cell penetrating peptide is a highly successful anticancer strategy that works by targeting the intracellular redox system. Pt-1-DMCa is an interesting candidate for a preclinical assessment of platinum-based therapy in GBM treatments and possibly other cancer types.










 Annuaire
Annuaire Contact
Contact Plan d'accès
Plan d'accès ENG
ENG Login
Login




