- ⌂
-
Le DPM
Les Equipements
L'Environnement
Zoom sur... Le Bâtiment André Rassat
Nommé d'après une figure tutélaire de la chimie grenobloise, ce bâtiment est recouvert d’une double peau en feuille métallique qui apporte une protection thermique sur 3 côtés et crée une unité architecturale favorisant l'intégration parmi les arbres du site.
-
Thématiques
Le Thème
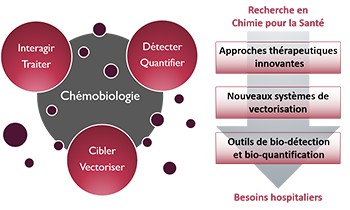
« Approches thérapeutiques innovantes »De nouvelles solutions thérapeutiques, de la cible biomacromolécu-laire émergente aux approches originales pour traiter les maladies
Le Thème
« Nouveaux systèmes de vectorisation »Combiner les propriétés d’inclusion de principes actifs, de franchisse-ment de barrières, d’adressage et de relar-gage en milieu vivant
Le Thème
« Outils de bio-détection et bio-quantification »Des dispositifs analytiques originaux pour la détection de cibles, de l’ion au micro-organisme en milieu complexe
Zoom sur... La Chémobiologie
-
Équipes
L'Équipe « COMET »
« COMET » développe la conception rationnelle, la synthèse et/ou l'extraction de composés à forte diversité/complexité comme nouveaux agents thérapeutiques et outils moléculaires pour la pénétration cellulaire ou la détection de biomolécules, actifs in vivo.L'Équipe « NOVA »
« NOVA » utilise des acides nucléiques fonctionnels comme éléments de reconnaissance pour des applications thérapeutiques ou diagnostiques, comme la sélection d'oligonucléotides, ou le développement de dispositifs d'analyses et de nanovecteurs.Les Services
-
Productions
Les Publications
La Vulgarisation
Les JSM
Zoom sur... La 12ème JSM (15 juin 2023)
Le DPM organise des journées scientifiques consacrées au médicament. L'objectif est de rassembler les spécialistes académiques et industriels autour d'une thématique. 2023 : Apports de la Chimie Click et de la Lumière en Chemobiologie
-
Partenariats
Les Formations
Les Consortiums
Les Financements
Zoom sur... L'environnement Grenoblois
Le DPM est un acteur central sur le bassin grenoblois en chimie, biologie et santé, lié au CHU Grenoble Alpes et à de nombreuses autres organisations : Pole de Recherche CBS, ICMG, Labex ARCANE, EUR CBH, Institut Carnot Polynat, Réseau GREEN.
Article
- Projet
- Luc CHOISNARD,
- Titre
- The water test could be a good indication to evaluate the effectiveness of the sterile barrier system of rigid sterilization containers.
-
[Full paper
 ]
] - Auteurs
- L. Decarout, C. Lambert, L. Choisnard, C. Andonian, C. Fayard
- Edition
- Annales Pharmaceutiques Françaises, 2018, 76(6), 464-472
- Année
- 2018
- Résumé
- The objective is to check the maintaining performance of filter barrier in sterilizations containers with the leakage rupture the junction tank/lid of the container. In order to control the good sealing performance, we use the water leak test as described in the normative document AFNOR FD S98-053. Does the leakage rupture cause the contamination of containers during the storage or transport between two sterilization units? Method. — We use 5 sets of 3 different containers (the first without barrier system (positive control), the others with barrier system (the second with positive water test and the third with negative water test)). The containers are sterilized after having positioned triptyc soy agar (TSA) plates at the bottom. In a closed chamber test, the containers are exposed with an aerosol of Micrococcus luteus under stress of an overpressure (25, 50 or 75 mbar) simulating atmospheric pressure variations. Each triptyc soy agar (TSA) plates are incubated during 5 days at 37◦C for bacterial identification. Results. — At 25 mbar, 74% of containers having a leakage with a water test let the microorganisms ingress despite the filter barrier. The results confirm that the overpressure increases the level of bacteria in containers. Furthermore, the containers with positive water test are more contaminated than containers with perfect sealing. The effectiveness of the sterile barrier system is evaluated by the logarithmic reduction value (LRV).




 Annuaire
Annuaire Contact
Contact Plan d'accès
Plan d'accès ENG
ENG Login
Login



