- ⌂
-
Le DPM
Les Equipements
L'Environnement
Zoom sur... Le Bâtiment André Rassat
Nommé d'après une figure tutélaire de la chimie grenobloise, ce bâtiment est recouvert d’une double peau en feuille métallique qui apporte une protection thermique sur 3 côtés et crée une unité architecturale favorisant l'intégration parmi les arbres du site.
-
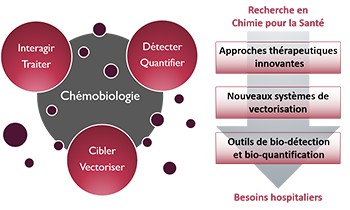
Thématiques
Le Thème
« Approches thérapeutiques innovantes »De nouvelles solutions thérapeutiques, de la cible biomacromolécu-laire émergente aux approches originales pour traiter les maladies
Le Thème
« Nouveaux systèmes de vectorisation »Combiner les propriétés d’inclusion de principes actifs, de franchisse-ment de barrières, d’adressage et de relar-gage en milieu vivant
Le Thème
« Outils de bio-détection et bio-quantification »Des dispositifs analytiques originaux pour la détection de cibles, de l’ion au micro-organisme en milieu complexe
Zoom sur... La Chémobiologie
-
Équipes
L'Équipe « COMET »
« COMET » développe la conception rationnelle, la synthèse et/ou l'extraction de composés à forte diversité/complexité comme nouveaux agents thérapeutiques et outils moléculaires pour la pénétration cellulaire ou la détection de biomolécules, actifs in vivo.L'Équipe « NOVA »
« NOVA » utilise des acides nucléiques fonctionnels comme éléments de reconnaissance pour des applications thérapeutiques ou diagnostiques, comme la sélection d'oligonucléotides, ou le développement de dispositifs d'analyses et de nanovecteurs.Les Services
-
Productions
Les Publications
La Vulgarisation
Les JSM
Zoom sur... La 12ème JSM (15 juin 2023)
Le DPM organise des journées scientifiques consacrées au médicament. L'objectif est de rassembler les spécialistes académiques et industriels autour d'une thématique. 2023 : Apports de la Chimie Click et de la Lumière en Chemobiologie
-
Partenariats
Les Formations
Les Consortiums
Les Financements
Zoom sur... L'environnement Grenoblois
Le DPM est un acteur central sur le bassin grenoblois en chimie, biologie et santé, lié au CHU Grenoble Alpes et à de nombreuses autres organisations : Pole de Recherche CBS, ICMG, Labex ARCANE, EUR CBH, Institut Carnot Polynat, Réseau GREEN.
Article
- Projet
- Basile PERES, Emile ROUSSEL,
- Titre
- Optimization of the Chromone Scaffold through QSAR and Docking Studies: Identification of Potent Inhibitors of ABCG2
-
[Full paper
 ]
] - Auteurs
- E. Roussel, V.-K. Tran-Nguyen, K. Bouhedjar, M.-A. Dems, A. Belaidi, B. Matougui, B. Peres, A. Azioune, O. Renaudet, P. Falson and A. Boumendjel
- Edition
- Eur. J. Med. Chem. 2019, 184, 111772
- Année
- 2019
- Résumé
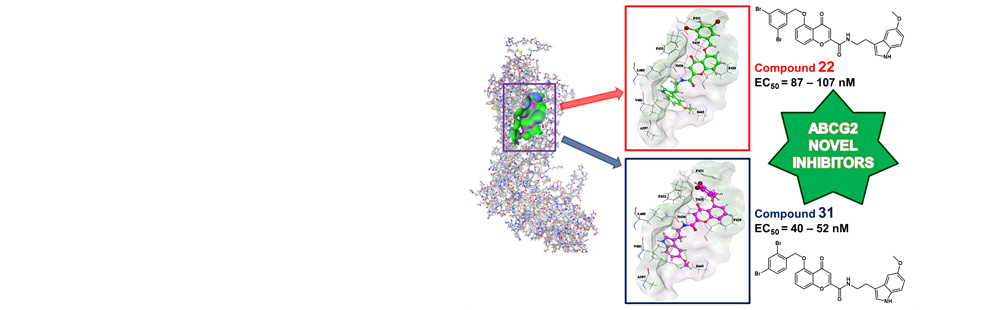
- The membrane transporter BCRP/ABCG2 has emerged as a privileged biological target for the development of small compounds capable of abolishing multidrug resistance. In this context, the chromone skeleton was found as an excellent scaffold for the design of ABCG2 inhibitors. With the aims of optimizing and developing more potent and selective modulators of the transporter, we herewith propose a multidisciplinary medicinal chemistry approach performed on this promising scaffold. A quantitative structure-activity relationship (QSAR) study on a series of chromone derivatives was first carried out, giving a robust model that was next applied to the design of 13 novel compounds derived from this nucleus. Two of the most active according to the model’s prediction (compounds 22 and 31) were synthesized and had their biological potency evaluated by experimental assays, confirming their high inhibitory activity against ABCG2 (experimental EC50 below 0.10 µM). A supplementary docking study was then conducted on the newly designed derivatives, proposing possible binding modes of these novel molecules in the putative ligand-binding site of the transporter and explaining why the two aforementioned compounds exerted the best activity according to biological data. Results from this study are recommended as references for further research in hopes of discovering new potent inhibitors of ABCG2.




 Annuaire
Annuaire Contact
Contact Plan d'accès
Plan d'accès ENG
ENG Login
Login



