- ⌂
-
Le DPM
Les Equipements
L'Environnement
Zoom sur... Le Bâtiment André Rassat
Nommé d'après une figure tutélaire de la chimie grenobloise, ce bâtiment est recouvert d’une double peau en feuille métallique qui apporte une protection thermique sur 3 côtés et crée une unité architecturale favorisant l'intégration parmi les arbres du site.
-
Thématiques
Le Thème
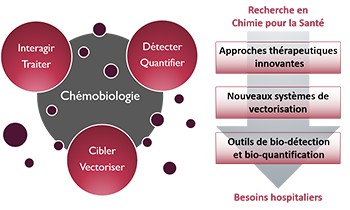
« Approches thérapeutiques innovantes »De nouvelles solutions thérapeutiques, de la cible biomacromolécu-laire émergente aux approches originales pour traiter les maladies
Le Thème
« Nouveaux systèmes de vectorisation »Combiner les propriétés d’inclusion de principes actifs, de franchisse-ment de barrières, d’adressage et de relar-gage en milieu vivant
Le Thème
« Outils de bio-détection et bio-quantification »Des dispositifs analytiques originaux pour la détection de cibles, de l’ion au micro-organisme en milieu complexe
Zoom sur... La Chémobiologie
-
Équipes
L'Équipe « COMET »
« COMET » développe la conception rationnelle, la synthèse et/ou l'extraction de composés à forte diversité/complexité comme nouveaux agents thérapeutiques et outils moléculaires pour la pénétration cellulaire ou la détection de biomolécules, actifs in vivo.L'Équipe « NOVA »
« NOVA » utilise des acides nucléiques fonctionnels comme éléments de reconnaissance pour des applications thérapeutiques ou diagnostiques, comme la sélection d'oligonucléotides, ou le développement de dispositifs d'analyses et de nanovecteurs.Les Services
-
Productions
Les Publications
La Vulgarisation
Les JSM
Zoom sur... La 12ème JSM (15 juin 2023)
Le DPM organise des journées scientifiques consacrées au médicament. L'objectif est de rassembler les spécialistes académiques et industriels autour d'une thématique. 2023 : Apports de la Chimie Click et de la Lumière en Chemobiologie
-
Partenariats
Les Formations
Les Consortiums
Les Financements
Zoom sur... L'environnement Grenoblois
Le DPM est un acteur central sur le bassin grenoblois en chimie, biologie et santé, lié au CHU Grenoble Alpes et à de nombreuses autres organisations : Pole de Recherche CBS, ICMG, Labex ARCANE, EUR CBH, Institut Carnot Polynat, Réseau GREEN.

Jean-Luc DECOUT
Professeur émérite au DPM depuis le 01-09-1998
Bureau : E304
rf@.@sepla-elbonerg-vinu@@@tuoced@.@cul-naej@
☏ 04 76 63 53 17
Projets
-
Drug design rationnel et cibles thérapeutiques complexes. Certaines cibles thérapeutiques sont complexes à modéliser, et nécessitent une expertise et des méthodes de calcul avancées. Leur étude requiert des associations innovantes entre chimie médicinale, RMN et calculs théoriques. L'objectif du projet est le développement de nouveaux inhibiteurs de métalloenzymes, d'interactions protéine-protéine, ou de multiples cibles, conçus de manière rationnelle.
Publications
-
Article • A Dual-Affinity Peptide Nucleic Acid for Targeting miRNA-21 Precursor Rescues Tumor Repressor PTEN Expression [Full paper
 ]
] Lian, Y.; Wang, A.; Lin, R.; Ke, X.; Zhan, X.; Lu, R.; Qian, T.; Ghosh, A.; Ong, A. A. L.; Toh, D.-F. K.; Patil, K. M.; Krishna, M. S.; Dezanet, C.; Decout, J.-L.; Maiti, S.; Wang, H.; Chen, G.
Cell Reports Physical Science 2025, 6 (11), 102935
-
Article • ABCB4 Disease-Causing Variants S242R, S346I, T437I and T1077M Significantly Impair Its Function and Display Differential Sensitivity to Potentiators [Full paper
 ]
] Madry, C.; Elbahnsi, A.; Delaunay, J.-L.; Stary, A.; Lagaye, S.; Couvert, P.; Corpechot, C.; Lemoinne, S.; Chignard, N.; Boucherle, B.; Gautheron, J.; Décout, J.-L.; Callebaut, I.; Aït-Slimane, T.
Sci Rep 2025, 15 (1), 44544
-
Article • Pharmacological Chaperones Improve Intra-Domain Stability and Inter-Domain Assembly via Distinct Binding Sites to Rescue Misfolded CFTR [Full paper
 ]
] N. Baatallah, A. Elbahnsi, J.-P. Mornon, B. Chevalier, I. Pranke, N. Servel, R. Zelli, J.-L. Decout, A. Edelman, I. Sermet-Gaudelus, I. Cellebaut, A. Hinzpeter
Cell. Mol. Life Sci. 2021, 78, 7813-7829
-
Article • Interest of Homodialkyl Neamine Derivatives against Resistant P. Aeruginosa, E. Coli, and β-Lactamases-Producing Bacteria—Effect of Alkyl Chain Length on the Interaction with LPS [Full paper
 ]
] J. Swain, C. Dezanet, H. Chalhoub, M. Auquière, J. Kempf, J.-L. Décout, M.-P. Mingeot-Leclercq
Int. J. Mol. Sci. 2021, 22, 8707
-
Patent • Method for synthesizing diversely substituted purines
Jean-Luc Decout, Renaud Zelli, Wael Zeinyeh, Benjamin Boucherle, Romain Haudecoeur
2021 •
-
Article • Targeting different binding sites in the CFTR structures allows to synergistically potentiate channel activity [Full paper
 ]
] L. Froux, A. Elbahnsi, B. Boucherle, A. Billet, N. Baatallah, B. Hoffmann, J. Alliot, R. Zelli, W. Zeinyeh, R. Haudecoeur, A. Fortuné, S. Mirval, C. Simard, P. Lehn, J.-P. Mornon, I. Callebaut, J.-L. Décout
Eur. J. Med. Chem. 2020, 190, 112116
-
Patent • Compounds for treating cystic fibrosis
Isabelle Callebaut, Jean-paul Mornon, Jean-Luc Decout, Frederic Becq, Pierre Lehn, Brice Hoffman, Benjamin Boucherle, Romain Haudecoeur, Antoine Fortune, Clement Boinot, Julien Alliot
2019 •
-
Article • Purine Chemistry in the Early RNA World at the Origins of Life: From RNA and Nucleobases Lesions to Current Key Metabolic Routes [Full paper
 ]
] Decout, J.-L.; Maurel, M.-C.
ChemBioChem 2025, 26 (11), e202500035
-
Article • A One-Pot Synthesis of Highly Functionalized Purines [Full paper
 ]
] R. Zelli, W. Zeinyeh, R. Haudecoeur, J. Alliot, B. Boucherle, I. Callebaut, J.-L. Decout
Org. Lett. 19, 2017, 23, 6360-6363
-
Article • A new straightforward synthesis of 2’, 3’-didehydro-2’, 3’-dideoxy-2’-(2”- (trimethylsilyl)ethylthio)thymidine, key intermediate for the synthesis of 2’-substituted thionucleosides
Maralise P. Oliveira, Lucas L. Franco, Maria C. A. Lima, Cláudia M. O. Simões, Suely L. Galdino, Ivan R. Pitta, Jean-Luc Décout, Ricardo J. Alves
J. Braz. Chem. Soc. 26, 816-821, 2015
-
Article • New 7-methylguanine derivatives targeting the influenza PB2 cap-binding domain. [Full paper
 ]
] S. Pautus, P. Sehr, J. Lewis, A. Fortuné, A. Wolkerstorfer, O. Szolar, D. Guilligay, T. Lunardi, J.-L. Decout, S. Cusack.
J. Med. Chem. 2013, 56, 8915-8930.
-
Article • Anti-retroviral and cytostatic activity of 2',3'-dideoxyribonucleotide 3'-disulfides.
B. Gerland, J. Desire, J. Balzarini, J.-L. Decout.
Bioorg. Med. Chem. 2008, 16, 6824-6831.
-
Article • Direct preparation of nucleoside vinyl disulfides from 2-(trimethylsilyl)ethyl sulfides, an access to vinylthiols.
B. Gerland, J. Desire, M. Lepoivre, J.-L. Decout
Org. Lett. 9, 2007, 3021-3023.
-
Patent • Process for the preparation of nucleoside disulfides and thiosulfinates
J.-L. Decout, B. Gerland, J. Desire
2006 • Université de Grenoble I / CNRS
-
Patent • Preparation of thionucleosides as antiviral and antitumor agents
J.-L. Decout, B. Gerland, J. Desire, M. Lepoivre, J. Balzarini.
2006 • Université de Grenoble I / CNRS
-
Article • Sugar-Modified Thionucleosides: Chemistry and Inhibition of Ribonucleotide Reductases and S-Adenosyl-L-Homocysteine Hydrolases.
J.-L. Decout, S. F. Wnuk
Frontiers in Nucleosides and Nucleic Acids Ed. by RF Schinazi and DC Liotta, 2004 IHL Press
-
Book • Frontiers in Nucleosides and Nucleic Acids
Sugar-Modified Thionucleosides: Chemistry and Inhibition of Ribonucleotide Reductases and S-Adenosyl-L-Homocysteine Hydrolases.
J.-L. Decout, S. F. Wnuk
2004 • 235-266, IHL Press - Tucker (GA), USA
-
Article • Deoxyribonucleoside 2’- or 3’-mixed disulfides: prodrugs to target ribonucleotide reductase and/or to inhibit HIV reverse transcription
B. Roy, S. Chambert, M. Lepoivre, A.-M. Aubertin, J. Balzarini, J.-L. Decout
J. Med. Chem. 2003, 46, 2565-2568
-
Article • Thionucleotides as inhibitors of ribonucleotide reductase
B. Roy, S. Chambert, M. Lepoivre, J.-L. Decout
Nucleosides Nucleotides Nucleic Acids 2003, 22, 883-885.
-
Article • Recent developments in the synthesis, the chemical modifications and the biological applications of sulfur modified nucleosides, nucleotides and oligonucleotides.
S. Chambert, J.-L. Decout
Org. Prep. Proc. Int. 2002, 34, 27-85.
-
Article • 2-Trimethylsilylethyl sulfides in the von Braun cyanogen bromide reaction : selective preparation of thiocyanates and application to nucleoside chemistry.
S. Chambert, F. Thomasson, J.-L. Decout
J. Org. Chem. 2002, 67, 1898 - 1904.
-
Article • The 2-(trimethylsilyl)ethyl sulfur group in synthesis
S. Chambert, J. Desire, J.-L. Decout
Synthesis 2002, 16, 2319-2334 (revue)
-
Article • Combining theoretical and experimental data to decipher CFTR 3D structures and functions
B. Hoffmann, A. Elbahnsi, P. Lehn, J.-L. Décout, F. Pietrucci, J.-P. Mornon, I. Callebaut
Cell. Mol. Life Sci. 2018, in press.
-
Oral Comm • Une approche rationnelle pour la thérapie de la mucoviscidose
Benjamin Boucherle
-
Oral Comm • Discovery of novel tailored F508del-CFTR binder correctors based on 3D structure models of entire CFTR protein for treating Cystic Fibrosis
Clément Boinot, Mathilde Jollivet Souchet, Brice Hoffmann, Jean-Paul Mornon, Benjamin Boucherle, Antoine Fortuné, Jean-Luc Decout, Pierre Lehn, Isabelle Callebaut, Frédéric Becq
-
Oral Comm • Models of the 3D structure of CFTR: from the understanding of the protein functions to the design of correctors
Brice Hoffmann, Jean-Paul Mornon, Benjamin Boucherle, Antoine Fortuné, Romain Haudecoeur, Clément Boinot, Mathilde Jollivet, Jean-Luc Decout, Frédéric Becq, Pierre Lehn, Isabelle Callebaut
-
Article • A new 9-alkyladenine-cyclic methylglyoxal diadduct activates wt- and F508del-cystic fibrosis transmembrane conductance regulator (CFTR) in vitro and in vivo.
B. Boucherle, J. Bertrand, B. Maurin, B.-L. Renard, A. Fortuné, B. Tremblier, F. Becq, C. Norez, J.-L. Decout.
Eur. J. Med. Chem. 2014, 83, 455-465.
-
Patent • Compounds for treating cystic fibrosis
Isabelle Callebaut, Jean-Paul Mornon, Jean-Luc Decout, Frédéric Becq, Pierre Lehn, Brice Hoffmann, Benjamin Boucherle, Romain Haudecoeur, Antoine Fortuné, Clément Boinot, Julien Alliot
2014 •
-
Article • In vivo study and validation of novel pharmacological modulators of water and ionic intestinal secretion for future therapeutic applications.
L. Dannhoffer, J.-L. Decout, F. Becq.
Fund. Clin. Pharmacol. 2011, 25, 79.
-
Article • An expeditious access to 5-pyrimidinol derivatives from cyclic methylglyoxal diadducts, formation of argpyrimidines under physiological conditions and discovery of new CFTR inhibitors.
B.-L. Renard, B. Boucherle, B. Maurin, M.-C. Molina, C. Norez, F. Becq, J.-L. Decout.
Eur. J. Med. Chem. 2011, 46, 1935-1941.
-
Article • Identification of a novel water soluble activator of wild-type and F508del CFTR: GPact-11a.
J. Bertrand, B. Boucherle, A. Billet, P. Melin-Heschel, L. Dannhoffer, C. Vandebrouck, C. Jayle, C. Routaboul, M.-C. Molina, J.-L. Decout, F. Becq, C. Norez.
Eur. Respir. J. 2010, 36, 311-322.
-
Oral Comm • Pharmacological characterization of a novel water-soluble activator of F508del-CFTR
J. Bertrand, B. Boucherle, L. Dannhoffer, P. Melin, C. Routaboul, J. L. Décout, F. Becq, C. Norez
-
Oral Comm • Activation of chloride secretion in human airway epithelial cells by a new pyrrolo[2,3-b]pyrazine
L. Dannhoffer, P. Melin, C. Faveau, B. Boucherle, C. Jayle, P. Corbi, Y. Mettey, J. L. Décout, F. Becq
-
Article • Discovery of alpha-aminoazaheterocycle-methylglyoxal adducts as a new class of high affinity inhibitors of CFTR chloride channels.
C. Routaboul, C. Norez, P. Melin, M.-C. Molina, B. Boucherle, F. Bossard, S. Noel, R. Robert, C. Gauthier, F. Becq, J.-L. Decout.
J. Pharmacol. Exp. Ther. 2007, 322, 1023-1035.
-
Patent • Zwitterionic CFTR channel modulators, preparation, pharmaceutical compositions and therapeutic use.
J.-L. Decout, C. Routaboul, F. Becq, C. Norez
2003 • Université Grenoble I / CNRS / Université de Poitiers
-
Article • New stereoselective reaction of methylglyoxal with 2-aminopyridines and adenine derivatives : formation of imino acid-nucleic base derivatives in water under mild conditions.
C. Routaboul, L. Dumas, I. Gautier-Luneau, J. Vergne, M.-C. Maurel, J.-L. Decout
Chem. Commun. 2002, 1114-1115.
-
Article • Effect of cardiolipin on the antimicrobial activity of a new amphiphilic aminoglycoside derivative on Pseudomonas aeruginosa
J. Swain, M. El Khoury, J. Kempf, F. Briée, P. Van Der Smissen, J.-L. Décout, M.-P. Mingeot-Leclercq
PLoS One 2018, 13, e0201752
-
Article • Broad-spectrum antibacterial amphiphilic aminoglycosides: A new focus on the structure of the lipophilic groups extends the series of active dialkyl neamines
L. Zimmermann, J. Kempf, F. Briée, J. Swain, M.-P. Mingeot-Leclercq, J.-L. Décout
Eur. J. Med. Chem. 2018, in press.
-
Article • New broad-spectrum antibacterial amphiphilic aminoglycosides active against resistant bacteria: From neamine derivatives to smaller neosamine analogues. [Full paper
 ]
] L. Zimmermann, I. Das, J. Desire, G. Sautrey, V. Barros R. S., M. El Khoury, M.-P. Mingeot-Leclercq, J.-L. Decout
J. Med. Chem. 2016, 59, 9350-9369
-
Article • New amphiphilic neamine derivatives active against resistant Pseudomonas aeruginosa and their interactions with lipopolysaccharides [Full paper
 ]
] Guillaume Sautrey, Louis Zimmermann, Magali Deleu, Alicia Delbar, Luiza Souza Machado, Katy Jeannot, Françoise Van Bambeke, Julien M. Buyck, Jean-Luc Decout, Marie-Paule Mingeot-Leclercq
Antimicrob. Agents Chemother. 2014, 58, 4420-4430.
-
Article • Tuning the antibacterial activity of amphiphilic neamine derivatives and comparison to paromamine homologues. [Full paper
 ]
] L. Zimmermann, A. Bussiere, M. Ouberai, I. Baussanne, C. Jolivalt, M.-P. Mingeot-Leclercq, J.-L. Decout.
J. Med. Chem. 2013, 56, 7691-7705.
-
Article • Antibiotic drugs aminoglycosides cleave DNA at abasic sites: Shedding new light on their toxicity? [Full paper
 ]
] M. Perigolo, J.-F. Constant, M. Peuchmaur, I. Pitta, J.-L. Decout.
Chem. Res. Tox. 2013, 26, 1710–1719.
-
Article • A Peptide Nucleic Acid-aminosugar conjugate targeting transactivation response element of HIV-1 RNA genome shows a high bioavailability in human cells and strongly inhibits tat-mediated transactivation of HIV-1. [Full paper
 ]
] I. Das, J. Desire, D. Manvar, I. Baussanne, V. N. Pandey, J.-L. Decout.
J. Med. Chem. 2012, 55, 6021-6032.
-
Article • The Pseudomonas aeruginosa membranes: a target for a new amphiphilic aminoglycoside derivative?
M. Ouberai, F. El Garch, A. Bussiere, M. Riou, D. Alsteens, L. Lins, I. Baussanne, Y. F. Dufrene, R. Brasseur, J.-L. Decout, M.-P. Mingeot-Leclercq.
BBA - Biomembranes 2011, 1808, 1716–1727.
-
Article • Synthesis and antimicrobial evaluation of amphiphilic neamine derivatives.
I. Baussanne, A. Bussiere, S. Halder, C. Ganem-Elbaz, M. Ouberai, M. Riou, J.-M. Paris, E. Ennifar, M.-P. Mingeot-Leclercq, J.-L. Decout.
J. Med. Chem. 2010, 53, 119-127.
-
Patent • Derives de glucosamine et leur utilisation comme agents antibacteriens. Brevet français déposé le 9/11/2010 (Université de Grenoble/CNRS).
J.-L. Decout, I. Das, J. Desire, I. Baussanne, M.-P. Mingeot-Leclercq.
2010 • Université Joseph Fourier/CNRS
-
Article • Synthesis and transfection properties of a series of lipidic neamine derivatives.
T. Le Gall, I. Baussanne, S. Halder, N. Carmoy, T. Montier, P. Lehn, J.-L. Decout.
Bioconjugate Chem. 2009, 20, 2032-2046.
-
Article • Unusual addition of amines to C-2 of vinyl sulfone-modified-β-D-pent-2-enofuranosyl carbohydrates: synthesis of a new class of β-anomeric D-arabino-2,3-Dideoxy-2-aminofuranosides.
I. Das, C. G. Suresh, J.-L. Decout, T. Pathak.
Carbohydrate Res. 2008, 343, 1287-1296.
-
Patent • Nouveaux dérivés de la néamine, méthode de préparation et propriétés antibiotiques.
J.-L. Decout, I. Baussanne, J. Desire, J.-M. Paris.
2008 • Université Joseph Fourier/CNRS
-
Article • A structure-based approach for targeting the HIV-1 genomic RNA dimerization initiation site.
E. Ennifar, J.-C. Paillart, S. Bernacchi, P. Walter, P. Pale, J.-L. Decout, R. Marquet, P. Dumas.
Biochimie 2007, 89, 1195-1203
-
Article • Mechanism of RNA cleavage catalyzed by sequence specific polyamide nucleic acid-neamine conjugate.
B. Chaubey, S. Tripathi, J. Desire, I. Baussanne, J.-L. Decout, V. Pandey.
Oligonucleotides 2007, 17, 302-313.
-
Article • Neamine dimers targeting the HIV-1 TAR RNA.
E. Riguet, J. Désiré, O. Boden, V. Ludwig, M. Göbel, C. Bailly, J.-L.Decout
Bioorg. Med. Chem. Lett. 2005, 15, 4651-4655.
-
Article • A route for preparing new neamine derivatives targeting HIV-1 TAR RNA.
E. Riguet, J. Desire, C. Bailly, J.-L. Decout
Tetrahedron 2004, 60, 8053-8064.
-
Article • A peptide nucleic acid-neamine conjugate that targets and cleaves HIV-1 TAR RNA inhibits viral replication.
E. Riguet, S. Tripathi, B. Chaubey, J. Desire, V. N. Pandey, J.-L. Decout.
J. Med. Chem. 2004, 47, 4806-4809.
-
Patent • PNA-neamine conjugates and methods for producing and using the same.
J.-L. Decout, V. N. Pandey, E. Riguet
2003 • University of Medicine and Dentistry of New Jersey, Newark, USA / Université de Grenoble I / CNRS
-
Article • Deficient Pseudomonas aeruginosa in MlaA/VacJ outer membrane lipoprotein shows decrease in rhamnolipids secretion, motility, and biofilm formation, and increase in fluoroquinolones susceptibility and innate immune response [Full paper
 ]
] M. Kaur, J. M. Buyck, F. Goormaghtigh, J. -L. Decout, N. Mozaheb, M. -P. Mingeot-Leclercq
Res. Microbiol. 2023, 174, 104132
-
Article • An Evolutionary Conserved Detoxification System for Membrane Lipid–Derived Peroxyl Radicals in Gram-Negative Bacteria [Full paper
 ]
] Marwa Naguib, Nicolás Feldman, Paulina Zarodkiewicz, Holly Shropshire, Christina Biamis, Omar M. El-Halfawy, Julia McCain, Clément Dezanet, Jean-Luc Décout, Yin Chen, Gonzalo Cosa, Miguel A. Valvano
PLOS Biol. 2022, 20, e3001610
-
Article • In Vitro Rescue of the Bile Acid Transport Function of ABCB11 Variants by CFTR Potentiators [Full paper
 ]
] Elodie Mareux, Martine Lapalus, Amel Ben Saad, Renaud Zelli, Mounia Lakli, Yosra Riahi, Marion Almes, Manon Banet, Isabelle Callebaut, Jean-Luc Decout, Thomas Falguières, Emmanuel Jacquemin, Emmanuel Gonzales
Int. J. Mol. Sci. 2022, 18, 10758
-
Article • A short chemically modified dsRNA-binding PNA (dbPNA) inhibits influenza viral replication by targeting viral RNA panhandle structure [Full paper
 ]
] J. Kesy, K. M. Patil, S. R. Kumar, Z. Shu, H. Y. Yong, L. Zimmermann, A. A. Lerk Ong, D.-F. Kaixin Toh, M. S. Krishna, L. Yang, J.-L. Decout, D. Luo, M. Prabakaran, G. Chen, E. Kierzek
Bioconjugate Chem. 2019, 30, 931–943
-
Article • Major increase of the reactivity and selectivity in aminoglycoside O-alkylation due to the presence of fluoride ions. [Full paper
 ]
] O. Jackowski, A. Bussière, C. Vanhaverbeke, I. Baussanne, E. Peyrin, M.-P. Mingeot-Leclercq, J.-L. Decout.
Tetrahedron 2012, 68, 737-746.
-
Article • Chiral ligand-exchange chromatography of amino acids using porous graphitic carbon coated with a dinaphthyl derivative of neamine.
M. Zaher, C. Ravelet, I. Baussanne, A. Ravel, C. Grosset, J.-L Decout, E. Peyrin.
Anal. Bioanal. Chem. 2009, 393, 655-660.
-
Article • Enantioseparation by micellar electrokinetic chromatography using a ligand exchange-based chiral pseudostationary phase
M. Zaher, C. Ravelet, C. Vanhaverbeke, I. Baussanne, S. Perrier, J. Fize, J.-L. Decout, E. Peyrin
Electrophoresis, 2009, 30, 2869-2873
-
Article • Covalently bonded DNA aptamer chiral stationary phase for the chromatographic resolution of adenosine.
J. Ruta, C. Ravelet, J. Désiré, J.-L. Décout, E. Peyrin.
Anal. Bioanal. Chem. 2008, 390, 1051-1057.
-
Article • Copper(II) complexes of lipophilic aminoglycoside derivatives for the amino acid enantiomeric separation by ligand-exchange liquid chromatography. [Full paper
 ]
] M. Zaher, I. Baussanne, C. Ravelet, S. Halder, M. Haroun, J. Fize, J.-L. Decout, E. Peyrin.
J Chromatogr A. 2008, 1185, 291-295.
-
Article • Competitive affinity capillary electrophoresis assay based on a hybrid pre-incubation/on-capillary mixing format using an enantioselective aptamer as affinity ligand.
J. Ruta, C. Ravelet, I. Baussanne, J. Fize, J.-L. Decout, E. Peyrin.
J. Sep.Sci. 2008, 31, 2239-2243
-
Patent • Chiral selectors for separating enantiomers of a compound.
E. Peyrin, J.-L. Décout, C. Ravelet, I. Baussanne.
2007 • Université de Grenoble I/CNRS
-
Article • Aptamer-based enantioselective competitive binding assay for the trace enantiomer detection.
J. Ruta, C. Ravelet, I. Baussanne, J.-L. Decout, E. Peyrin.
Anal. Chem. 2007, 79, 4716-4719.
-
Article • Sequence-specific nucleic acid damage induced by “enzyme activable” peptide nucleic acid conjugates
P. Simon, J.-L. Decout, M. Fontecave
Angew. Chem. Int. Ed. 2006,45, 6859-6861.
-
Article • DNA detection through signal amplification using NADH:Flavin Oxidoreductase and oligonucleotide-flavin conjugates as cofactors
P. Simon, C. Dueymes, M. Fontecave, J.-L. Decout
Angew. Chem. Int. Ed. 2005, 44, 2764-2767.
-
Article • New flavin and deazaflavin oligonucleotide conjugates for the amperometric detection of DNA hybridization.
S. Cosnier, C. Gondran, C. Dueymes, P. Simon, M. Fontecave, J.-L. Decout
Chem. Commun. 2004, 1624-1625.
-
Article • Adenine-aptamer complexes : a bipartite RNA site which binds the adenine nucleic base.
M. Meli, J. Vergne, J.-L. Decout, M.C. Maurel
J. Biol. Chem. 2002, 277, 2104-2111.
-
Article • Fluorescent DNA microarrays using deazaflavin-oligonucleotide probes.
C. Dueymes, J.-L. Decout, P. Peltie, M. Fontecave
Angew. Chem. Int. Ed. 2002, 41, 486-489
-
Article • RNA-acting antibiotics: in vitro selection of RNA aptamers for the design of new bioactive molecules less susceptible to bacterial resistances
M.-C. Maurel, B. Biard, C. Moulinier, D. Braz, J. Nugier, I. Chaumas, M. Reboud-Ravaux, J-L. Decout
J. Pharm. Pharmacol. 2002, 67, 1898-1904 (revue)
-
Patent • Method and support for biological analysis using oligonucleotides comprising a marker capable of enzymatic activation.
J.-L. Decout, M. Fontecave, C. Dueymes
2001 • CEA / Université de Grenoble I
-
Patent • Analysis of biological targets using a biochip comprising a fluorescent marker
M. Cuzin, P. Peltie, M Fontecave, J.-L. Decout, C. Dueymes
2000 • CEA / Université de Grenoble I




 Annuaire
Annuaire Contact
Contact Plan d'accès
Plan d'accès ENG
ENG Login
Login

 Equipe « COMET »
Equipe « COMET »

