- ⌂
-
Le DPM
Les Equipements
L'Environnement
Zoom sur... Le Bâtiment André Rassat
Nommé d'après une figure tutélaire de la chimie grenobloise, ce bâtiment est recouvert d’une double peau en feuille métallique qui apporte une protection thermique sur 3 côtés et crée une unité architecturale favorisant l'intégration parmi les arbres du site.
-
Thématiques
Le Thème
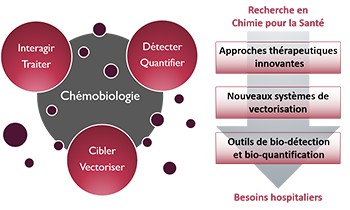
« Approches thérapeutiques innovantes »De nouvelles solutions thérapeutiques, de la cible biomacromolécu-laire émergente aux approches originales pour traiter les maladies
Le Thème
« Nouveaux systèmes de vectorisation »Combiner les propriétés d’inclusion de principes actifs, de franchisse-ment de barrières, d’adressage et de relar-gage en milieu vivant
Le Thème
« Outils de bio-détection et bio-quantification »Des dispositifs analytiques originaux pour la détection de cibles, de l’ion au micro-organisme en milieu complexe
Zoom sur... La Chémobiologie
-
Équipes
L'Équipe « COMET »
« COMET » développe la conception rationnelle, la synthèse et/ou l'extraction de composés à forte diversité/complexité comme nouveaux agents thérapeutiques et outils moléculaires pour la pénétration cellulaire ou la détection de biomolécules, actifs in vivo.L'Équipe « NOVA »
« NOVA » utilise des acides nucléiques fonctionnels comme éléments de reconnaissance pour des applications thérapeutiques ou diagnostiques, comme la sélection d'oligonucléotides, ou le développement de dispositifs d'analyses et de nanovecteurs.Les Services
-
Productions
Les Publications
La Vulgarisation
Les JSM
Zoom sur... La 12ème JSM (15 juin 2023)
Le DPM organise des journées scientifiques consacrées au médicament. L'objectif est de rassembler les spécialistes académiques et industriels autour d'une thématique. 2023 : Apports de la Chimie Click et de la Lumière en Chemobiologie
-
Partenariats
Les Formations
Les Consortiums
Les Financements
Zoom sur... L'environnement Grenoblois
Le DPM est un acteur central sur le bassin grenoblois en chimie, biologie et santé, lié au CHU Grenoble Alpes et à de nombreuses autres organisations : Pole de Recherche CBS, ICMG, Labex ARCANE, EUR CBH, Institut Carnot Polynat, Réseau GREEN.
Article
- Projet
- Yung-Sing WONG, Antoine FORTUNE, Aline THOMAS,
- Titre
- Chimeric Protein-Protein Interface Inhibitors Allow Efficient Inhibition of Type III Secretion Machinery and Pseudomonas aeruginosa Virulence
-
[Full paper
 ]
] - Auteurs
- Ngo, T.-D.; Plé, S.; Thomas, A.; Barette, C.; Fortuné, A.; Bouzidi, Y.; Fauvarque, M.-O.; Pereira de Freitas, R.; Francisco Hilário, F.; Attrée, I.; Wong, Y.-S.; Fraudry, E.
- Edition
- ACS Infect. Dis.
- Année
- 2019
- Résumé
- Pseudomonas aeruginosa (P. aeruginosa) is an opportunistic pathogen naturally resistant to many common antibiotics and acquires new resistance traits at an alarming pace. Targeting the bacterial virulence factors by an antivirulence strategy, therefore, represents a promising alternative approach besides antibiotic therapy. The Type III secretion system (T3SS) of P. aeruginosa is one of its main virulence factors. It consists of more than 20 proteins building a complex syringe-like machinery enabling the injection of toxin into host cells. Previous works showed that disrupting interactions between components of this machinery efficiently lowers the bacterial virulence. Using automated target-based screening of commercial and in-house libraries of small molecules, we identified compounds inhibiting the protein-protein interaction between PscE and PscG, the two cognate chaperones of the needle subunit PscF of P. aeruginosa T3SS. Two hits were selected and assembled using Split/Mix/Click chemistry to build larger hybrid analogues. Their efficacy and toxicity were evaluated using phenotypic analysis including automated microscopy and image analysis. Two nontoxic hybrid leads specifically inhibited the T3SS and reduced the ex vivo cytotoxicity of bacteria and their virulence in Galleria mellonella.




 Annuaire
Annuaire Contact
Contact Plan d'accès
Plan d'accès ENG
ENG Login
Login



