- ⌂
-
Le DPM
Les Equipements
L'Environnement
Zoom sur... Le Bâtiment André Rassat
Nommé d'après une figure tutélaire de la chimie grenobloise, ce bâtiment est recouvert d’une double peau en feuille métallique qui apporte une protection thermique sur 3 côtés et crée une unité architecturale favorisant l'intégration parmi les arbres du site.
-
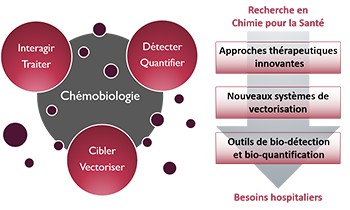
Thématiques
Le Thème
« Approches thérapeutiques innovantes »De nouvelles solutions thérapeutiques, de la cible biomacromolécu-laire émergente aux approches originales pour traiter les maladies
Le Thème
« Nouveaux systèmes de vectorisation »Combiner les propriétés d’inclusion de principes actifs, de franchisse-ment de barrières, d’adressage et de relar-gage en milieu vivant
Le Thème
« Outils de bio-détection et bio-quantification »Des dispositifs analytiques originaux pour la détection de cibles, de l’ion au micro-organisme en milieu complexe
Zoom sur... La Chémobiologie
-
Équipes
L'Équipe « COMET »
« COMET » développe la conception rationnelle, la synthèse et/ou l'extraction de composés à forte diversité/complexité comme nouveaux agents thérapeutiques et outils moléculaires pour la pénétration cellulaire ou la détection de biomolécules, actifs in vivo.L'Équipe « NOVA »
« NOVA » utilise des acides nucléiques fonctionnels comme éléments de reconnaissance pour des applications thérapeutiques ou diagnostiques, comme la sélection d'oligonucléotides, ou le développement de dispositifs d'analyses et de nanovecteurs.Les Services
-
Productions
Les Publications
La Vulgarisation
Les JSM
Zoom sur... La 12ème JSM (15 juin 2023)
Le DPM organise des journées scientifiques consacrées au médicament. L'objectif est de rassembler les spécialistes académiques et industriels autour d'une thématique. 2023 : Apports de la Chimie Click et de la Lumière en Chemobiologie
-
Partenariats
Les Formations
Les Consortiums
Les Financements
Zoom sur... L'environnement Grenoblois
Le DPM est un acteur central sur le bassin grenoblois en chimie, biologie et santé, lié au CHU Grenoble Alpes et à de nombreuses autres organisations : Pole de Recherche CBS, ICMG, Labex ARCANE, EUR CBH, Institut Carnot Polynat, Réseau GREEN.
Article
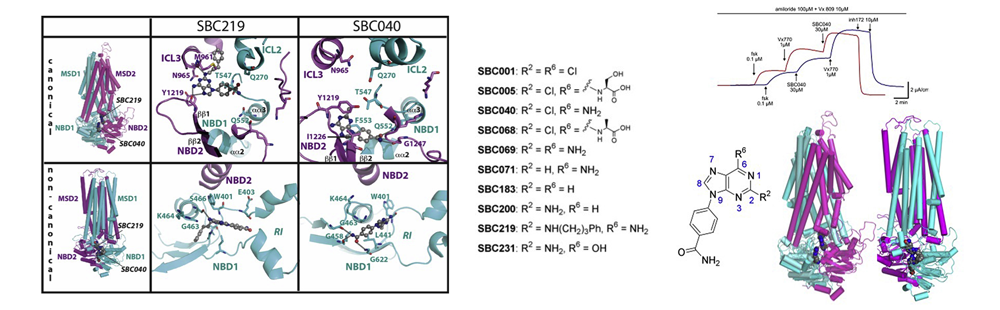
- Projet
- Drug design rationnel et cibles thérapeutiques complexes
- Jean-Luc DECOUT, Antoine FORTUNE, Benjamin BOUCHERLE, Romain HAUDECOEUR, Renaud ZELLI,
- Titre
- Targeting different binding sites in the CFTR structures allows to synergistically potentiate channel activity
-
[Full paper
 ]
] - Auteurs
- L. Froux, A. Elbahnsi, B. Boucherle, A. Billet, N. Baatallah, B. Hoffmann, J. Alliot, R. Zelli, W. Zeinyeh, R. Haudecoeur, A. Fortuné, S. Mirval, C. Simard, P. Lehn, J.-P. Mornon, I. Callebaut, J.-L. Décout
- Edition
- Eur. J. Med. Chem. 2020, 190, 112116
- Année
- 2020
- Résumé
- Recent evidence shows that combination of correctors and potentiators, such as the drug ivacaftor (VX-770), can significantly restore the functional expression of mutated Cystic Fibrosis Transmembrane conductance Regulator (CFTR), an anion channel which is mutated in cystic fibrosis (CF). The success of these combinatorial therapies highlights the necessity of identifying a broad panel of specific binding mode modulators, occupying several distinct binding sites at structural level. Here, we identified two small molecules, SBC040 and SBC219, which are two efficient cAMP-independent potentiators, acting at low concentration of forskolin with EC50 close to 1 μM and in a synergic way with the drug VX-770 on several CFTR mutants of classes II and III. Molecular dynamics simulations suggested potential SBC binding sites at the vicinity of ATP-binding sites, distinct from those currently proposed for VX-770, outlining SBC molecules as members of a new family of potentiators.




 Annuaire
Annuaire Contact
Contact Plan d'accès
Plan d'accès ENG
ENG Login
Login



